Cold Chain Logistics Grow Alongside Biologics
As researchers turned from one-size-fits-all medications to more targeted therapeutics, temperature and handling became more important. Biologics – therapeutics made from living cells – were developed and the number and type of vaccines also increased. These environmentally sensitive therapeutics make up about half of the drugs approved in the past several years, according to Pharmaceutical Commerce. Many are sensitive to temperature, vibrations and impacts, and need a robust cold chain logistics program so they can remain viable for patients.

Aside from biologicals, vaccines and blood products, however, relatively few pharmaceutical products require refrigerated or frozen temperatures. Most, in fact, most favor controlled room temperature (CRT) and, when protected from harsh temperatures, can be shipped as standard trucking or air freight. Like their highly sensitive cousins, though, CRT products are now monitored for temperature as they are shipped throughout the global pharmaceutical supply chain.
Cost-effective Monitoring Options for Healthcare Logistics
A variety of cost-effective monitoring options are available for healthcare logistics, from go/no go temperature indicators to temperature recorders featuring real-time reporting. Here are a few:
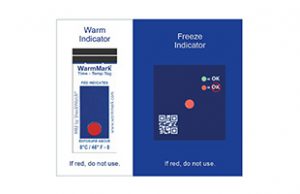
Cold Chain Complete Temperature Card, a go/no go freeze indicator coupled with a time-temperature warm indicator, changes color when it becomes too hot or too cold. It is good for cold chain logistics products that are sensitive to both heat and cold.
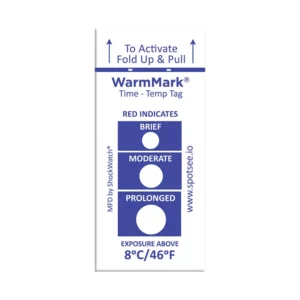
WarmMark® Time Temperature indicator is designed for products that must stay below a specific temperature. This indicator is available with threshold limits from -18°C/0°F all the way to 37°C/99°F. The 8°C / 46°F version is currently being used to monitor COVID-19 specimens in transport and storage. The WarmMark is a single-use temperature indicator that turns red when temperatures exceed the chosen threshold and also provides information regarding the duration of the temperature excursion.
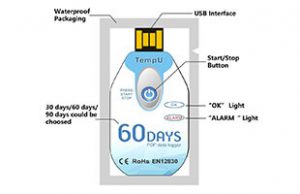
TempU Temperature Recorder is a good choice for refrigerated products that must stay between 2°C/35°F and 8°C/46°F. Designed for 60-day use, it can log 10,000 events. Excursions are shown by a flashing red light, and a complete PDF report can be downloaded at the end of a transport. Customization of the TempU for a product’s specific requirements is possible.
LOG-IC Temperature Recorder records temperatures between -80°C/-112° to 75°C/167°F so it is ideal for deep-frozen shipments as well as those likely to experience high heat (like those shipped to the Middle East). It features eight configurable alarm settings so quality and logistics teams can understand exactly what temperature exposure is being experienced by their product. Data can be downloaded over a USB port or from the semi-passive wireless RFID interface. The RFID interface enables data to be downloaded during shipments without the need to open the insulated shipper.
The ShockWatch RFID impact indicator is a good addition to shipments of biologics that are sensitive to impacts. These passive RFID indicators can help document chain of custody because their position and impact status is scanned into asset tracking systems each time they pass an RFID reader. When used with a temperature indicator, ShockWatch RFID tags provide a complete solution for cold chain/supply chain monitoring.
Pharmaceutical Supply Chain Monitors Enable Logistics Optimization and Accountability

Another learned about the perils of tarmacs on even balmy days. Changes to packaging and handling ensued. Problems like those contributed to a shortage of more than 200 medications in 2019, according to the American Society of Health-System Pharmacists. These aren’t the only disruptions a pharmaceutical supply chain can face. The FDA points out that drug shortages can be exacerbated by logistical challenges. They recommend pushing for better quality management by manufacturers to avoid supply chain mishaps caused by employees, weather, accidents, and geographical challenges.
Logistics condition monitors are a vital technology in the pharmaceutical supply chain that helps ensure product quality hasn’t deteriorated during shipping and, therefore, is safe to use. And, it provides invaluable insight into the supply chain so you can identify and resolve problems before they occur.
To find the controlled-temperature logistics solution best for you, get in touch with us.
Case Study
Whitepapers
Publications and Feature Stories
|
|
|
|
|
|







 ShockLog Satellite
ShockLog Satellite
