Saliva Indicators Case Study
Summary: Saliva testing is, in most cases, at least as effective as nasal swabs at diagnosing COVID-19. That’s why the FDA authorized five such kits as of August 2021, with others still in development. For saliva diagnostic kits to be accurate they must have enough saliva to test.
Product Solution: Saliva Indicator

Saliva Indicators Case Study
|
Company Profile Industry: Drug Testing Facilities Application: Testing of Saliva Challenge: Ensuring that drug tests are accurate |
 |
SpotSee Color-Change Saliva Indicators Help Minimize False Positives
Saliva testing is, in most cases, at least as effective as nasal swabs at diagnosing COVID-19. That’s why the FDA authorized five such kits as of August 2021, with others still in development. For saliva diagnostic kits to be accurate, however, they must have enough saliva to test.
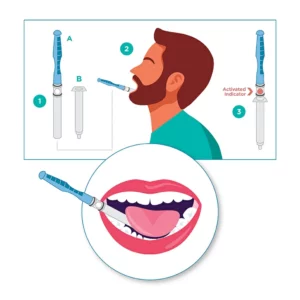
“Spit in a tube” tests can have a fill line so people being tested can add the needed quantities, but not all tests use that method. Some, for example, include a sponge-like substrate that absorbs the saliva in the mouth. This swab is then placed into a carrier and sent to a lab. The absorbent material enables it to be dispensed for testing simply by compression, extracting a droplet at a time. Consequently, one sample specimen can be tested for the presence of many substances.
That’s great for the laboratories analyzing the saliva, but not so great for the people being tested. The problem is that people can’t see whether the sponge absorbed enough saliva from their mouths to ensure accurate test results. With an inadequate quantity of saliva, false negatives may be returned and those who tested negative for the SARS-CoV-2 may carry – and therefore spread – the virus unknowingly. Collecting ample quantities of sample specimen reduces that risk.
Saliva Indicators:

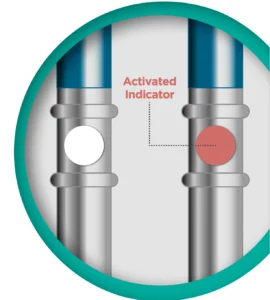
Saliva tests aren’t limited to COVID-19 testing, of course. They’re also used to test for drugs, alcohol, DNA, and certain hormones. These tests are used by state Highway Patrol departments, clinics, and health departments, and, particularly with COVID-19, for mail-in tests in which those being tested never see a healthcare provider or officer. Adding a hydrochromic indicator to the test swab – the material that collects and absorbs the saliva – eliminates the problem of inadequate sample quantities. Once the necessary quantity of sample is absorbed, the dot will change color to provide a readily evident, visual indicator.
Saliva kit manufacturers can incorporate this feature easily into their existing kits and workflows. They only need to add the adhesive indicator – a small, paper, color-change dot – to the absorbent material inside the dispensing unit before it is shipped. This can be done by standard operating machines or manually, just like adding a label.
SpotSee can design the saliva indicator dots in virtually any shape or color change combination customers want. This flexibility enables these dots (made from uncoated paper with adhesive backs) to work effectively with a variety of collection materials and form factors. For example, the dots can change from white to another color – red, for example – or from one color to another, and can be designed in any shape or size needed.
These hydrochromic saliva indicator dots are highly stable, with a 12-month shelf-life, which enables manufacturers to keep stock on hand in anticipation of demand.
Color-change saliva indicators provide an added level of efficacy for saliva kits and, by reducing false positives, can go a long way towards helping to contain the current pandemic and future outbreaks.

Contact us with any questions you have
about this case study or to schedule a
meeting with a SpotSee operations expert!

