Flu Vaccine Cold Chain Case Study: WarmMark
![]() Summary: The WarmMark temperature indicators trigger only when product temperatures have exceeded a specific temperature for a specific period of time. With at-a-glance ease, healthcare professionals can know instantly whether the vaccines maintained proper temperatures during shipping. These indicators give them added confidence that the vaccines have maintained their efficacy
Summary: The WarmMark temperature indicators trigger only when product temperatures have exceeded a specific temperature for a specific period of time. With at-a-glance ease, healthcare professionals can know instantly whether the vaccines maintained proper temperatures during shipping. These indicators give them added confidence that the vaccines have maintained their efficacy
Product Solution: WarmMark Temperature Indicator
Flu Vaccine Cold Chain Case Study: WarmMark
|
Company Profile
Application: Flu shot last mile shipping Challenge: Maintaining efficacy of flu vaccine during shipment from distribution centers to doctor’s offices, clinics and hospitals. |
 |
WarmMark Temperature Indicators Protect the Flu Vaccine Cold Chain as Vaccinations Surge
Nearly 194 million doses of the influenza vaccine were administered in the 2020/2021 flu season, according to the Center for Disease Control and Prevention (CDC), markedly higher than in previous seasons. If that vaccination trend continues, the 2021/2022 flu season may exceed 200 million influenza vaccinations for everyone six months of age and older.
With so many vaccine doses being shipped to pharmacies, physicians’ offices, and hospitals globally, there is a risk of mishaps. Temperature excursions are among the many things that can go wrong. Despite careful packaging in insulated cartons that have been certified and validated, vaccines lose their effectiveness if those cartons are damaged, if weather conditions change or shipping delays cause cold packs to thaw, or if routing mistakes occur.
“Potency is reduced every time a vaccine is exposed to an improper condition,” according to the CDC’s 2021 Vaccine Storage and Handling Toolkit. The harm isn’t necessarily visible, either, because the appearance of the vaccine often doesn’t change in relation to inappropriate temperatures. Without a time-temperature indicator inside the package, healthcare professionals have no easy way of knowing whether the vaccines experienced a temperature excursion.
Even once the vaccine shipments have arrived at healthcare facilities and been stored in purpose-built, pharmaceutical-grade refrigerators, they still may experience temperature excursions. For example, refrigerator door gaskets may not seal properly or refrigeration equipment may fail completely. Alternatively, the refrigerator may be placed near heating vents or in sunlight, causing internal temperatures to fluctuate and possibly damage the vaccines.

SpotSee’s WarmMark® is an ideal solution for monitoring vaccine temperature during shipping, and even during storage. This single-use, time-temperature indicator monitors for cumulative temperature excursions above a predetermined threshold.
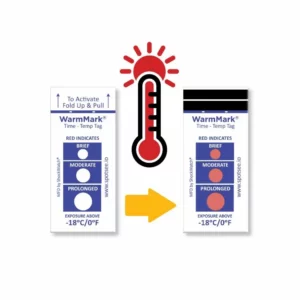
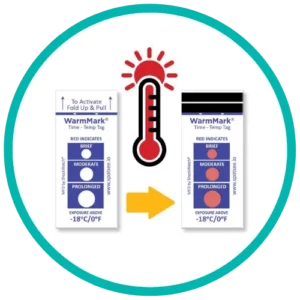
The WarmMark is available with thresholds between -18° and 37°C. When the cold chain is broken for a specific duration, the WarmMark windows change from white to red.
The WarmMark temperature indicators trigger only when product temperatures have exceeded a specific temperature for a specific period of time. With at-a-glance ease, healthcare professionals can know instantly whether the vaccines maintained proper temperatures during shipping. These indicators give them added confidence that the vaccines have maintained their efficacy.
WarmMark temperature indicators are easy for shippers to use, too. Simply pull the activation tab and the indicator is ready to go. Then place the WarmMark monitor inside the packaging as close to the product /vaccine as possible.
Using SpotSee’s WarmMark temperature indicators provide the assurance healthcare providers need during influenza season to ensure the vaccines they administrator are as safe and effective as when they left the manufacturer.
Contact us with any questions you have about this case study or to schedule a meeting with a SpotSee operations expert!

